- I globuli rossi influenzano il trasporto dei drug carrier nella microcircolazione
Sommario Nonostante i numerosi studi nel campo delle nanotecnologie per la progettazione di nano-terapeutici, le barriere biologiche al trasporto di farmaci incontrate nella circolazione sanguigna rappresentano un grande problema, impedendo l’efficiente trasporto del farmaco ai tessuti/organi danneggiati. Uno degli step fondamentali del viaggio della microparticella verso il target è il trasporto nella microcircolazione sanguigna, dove avviene l’interazione con le cellule del sangue.
Il sangue è un fluido non-Newtoniano composto principalmente di globuli rossi (GR), caratterizzati da un’elevata deformabilità (Tomaiuolo, Biomicrofluidics 2014). Nella microcircolazione (vasi con diametro inferiore ai 300 mm) i GR tendono ad addensarsi verso il centro del vaso sanguigno a causa di interazioni idrodinamiche con le pareti, lasciando vicino ad esse uno strato di plasma privo di cellule, detto cell-free layer (CFL) (Kumar and Graham, Soft Matter 2012). In analogia con globuli bianchi e piastrine, anche i drug carriers tendono ad accumularsi nel CFL (Tilles and Eckstein, Microvascular research 1987), in un fenomeno noto come marginazione, che rappresenta un tema centrale nell’ambito del drug delivery, in quanto il legame tra drug carriers e tessuti bersaglio può avvenire solo in seguito ad una diretta interazione tra particelle e pareti dei vasi.
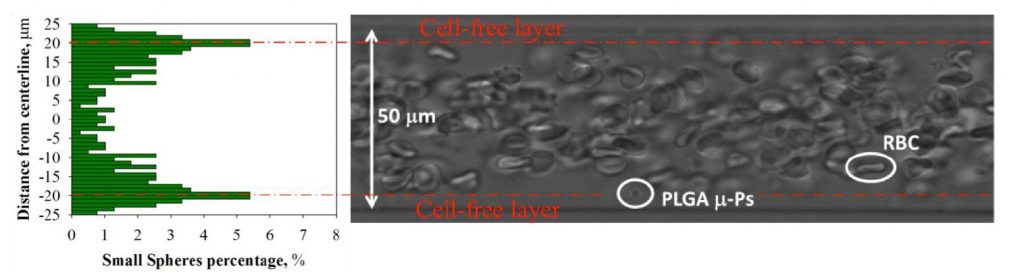
Qui, viene presentata una metodologia basata su tecniche microfluidiche, microscopia confocale ed analisi delle immagini finalizzata allo studio dell’influenza dei GR sulla marginazione di micro-particelle. Sono stati valutati i meccanismi che regolano il trasporto di GR e di particelle nella microcircolazione, valutando gli effetti del flusso di GR e delle caratteristiche delle particelle stesse (dimensione, forma, carica superficiale) sulla loro marginazione. E’ stato osservato che, in assenza di GR, le micro-particelle mostrano una distribuzione uniforme lungo il piano diametrale del capillare, muovendosi con la stessa velocità del fluido sospendente mentre, in presenza di GR, tendono a migrare verso le pareti del capillare, accumulandosi nel CFL (Fig.1) (D’Apolito et al., J Control Release 2015). In particolare, particelle sferiche di 3 μm mostrano una propensione alla marginazione superiore rispetto alle sfere da 1 μm, in accordo con lavori che confermano che la dimensione ottimale ai fini della marginazione è di circa 2-3 μm (Mueller et al. 2014). Inoltre, si è dimostrato che carrier sferici e discoidali hanno una maggiore propensione alla marginazione rispetto a quelli a bacchetta, e che la carica superficiale dei carrier non ne influenza la marginazione. Infine, si è visto che la percentuale di particelle sferiche nel CFL aumenta all’aumentare della shear rate (D’Apolito et al., Current drug delivery 2017).
Parole chiave marginazione, globuli rossi, drug delivery, microparticelle
Abstract Despite the efforts made in the design and functionalization of nanotherapeutics, the biological barriers to drug transport in microcirculation represent a big issue, preventing the delivery of drug to damaged organ/tissue. In fact, after the injection within blood stream and before reaching the diseased cells, drug carriers have to penetrate the barriers present in the body, undergoing a long journey, even defined as an odyssey in a recent paper. One of the key step of the journey is the transport in microcirculation, where drug carriers interact with blood cells. In fact, at the micro-scale of carriers, blood cannot be considered as a homogeneous fluid but as a concentrated suspension of red blood cells (RBCs), which represent the large majority of blood cells. RBCs are deformable objects (Tomaiuolo, Biomicrofluidics 2014) which, in the Poisueille flow occurring in microvessels, are pushed away from vessel wall by a wall-induced lift enhanced by their deformability. This effect is counterbalanced by the shear-induced diffusion that arises due to cell-cell collisions. This leads to RBC migration towards vessel centerline, creating a RBC-rich core in the center of the vessel and a cell-free layer (CFL) in proximity of the wall (Kumar and Graham, Soft Matter 2012; Tilles and Eckstein, Microvascular research 1987). In turn, the flow of drug carriers in the RBC-rich core is affected by their collisions with RBCs. Drug-carrier-RBC collisions cause carrier shear-induced slow diffusion in a size and shape dependent manner and, subsequently, a fast lateral displacement in the proximity of the CFL, called “waterfall effect”. The latter likely results from bypass regions through which particles can rapidly reach the CFL, which are related to the inhomogeneity of RBC spatial distribution. The combination of the slow shear induced diffusion and the fast waterfall effect leads to the phenomenon of margination.
Margination is the mechanism according to which particles migrate along vessel radius to the wall, in analogy with the behavior of platelets, which concentrate in the CFL near the wall.
Here, an in vitro systematic microfluidic investigation of RBCs in microconfined conditions is presented, focusing on the role played by RBC deformability on the interaction between flowing RBCs and drug carriers. It has been demonstrated that micro-drug carriers, when flowing with RBCs, tend to accumulate near the capillary wall. The dependence of micro-particle (m-Ps) distribution and delivery efficacy on the presence of RBCs, shear rate, particle size, shape, surface charge and stiffness has been examined. Thus, all types of m-Ps, in presence of RBCs, move at steady state with the same velocity of RBCs for a given value of the imposed shear rate. In other words, the velocity distribution along the capillary tube is governed by the presence of RBCs. Moreover, we show that margination, which is almost absent when particles are suspended in a cell-free medium, is drastically enhanced by RBCs (Fig.1) (D’Apolito et al., J Control Release 2015); we found, indeed, that most of the m-Ps lay in proximity of the walls. In particular, as the shear rate increases, the m-Ps concentration near the wall increases. The margination is also affected by m-Ps size and shape, larger spherical/discoid particles being more effectively marginated both in vitro and in vivo. m-Ps with different surface charge, instead, show a comparable margination propensity, suggesting that the presence of RBCs governs suspension flow behavior independently on m-Ps surface properties (D’Apolito et al., Current drug delivery 2017).
Keywords margination, red blood cells, drug delivery, micro-particles













